Androcar and progestin victims sue the state for compensation
After the mediation and the victims of Depakin, it is the turn of Androcar and other progestins (Lutenil, Lutheran, etc.) to demand responsibility from the state. According to information from the world, the first two requests were filed on March 7 before the Administrative Court of Montreul, in the department Seine-Saint-Denis, where the National Medicines Safety Agency (ANSM) is based. They are targeting the Ministry of Health to seek compensation of 748,777 euros and 361,515 euros respectively. About fifty appeals As of today 450 identified files are in preparation. They rely on medical expertise that questions the delayed reaction of health officials to properly report the meningioma (brain tumor) risks associated with these progesterone-derived hormonal treatments, despite warnings. When contacted, the Ministry of Health and ANSM did not want to comment.
Initially developed against hirsutism, Androcor (trade name for cyproterone acetate) is a drug that inhibits male hormones. Produced by Bayer Laboratories, marketed since 1980, it is widely prescribed to women for endometriosis, acne or as a contraceptive. It can also be given to transgender people for its antiandrogenic properties.
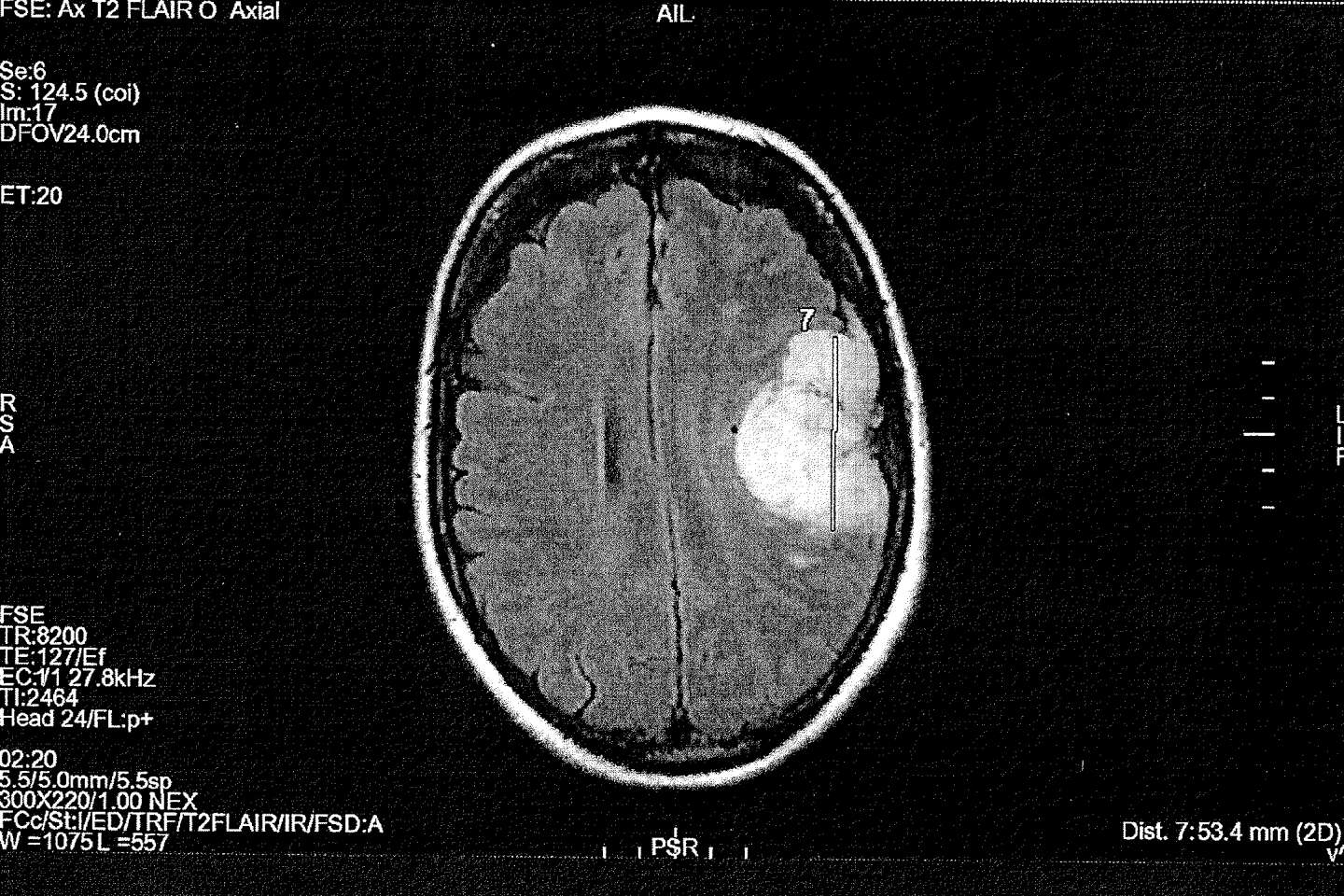
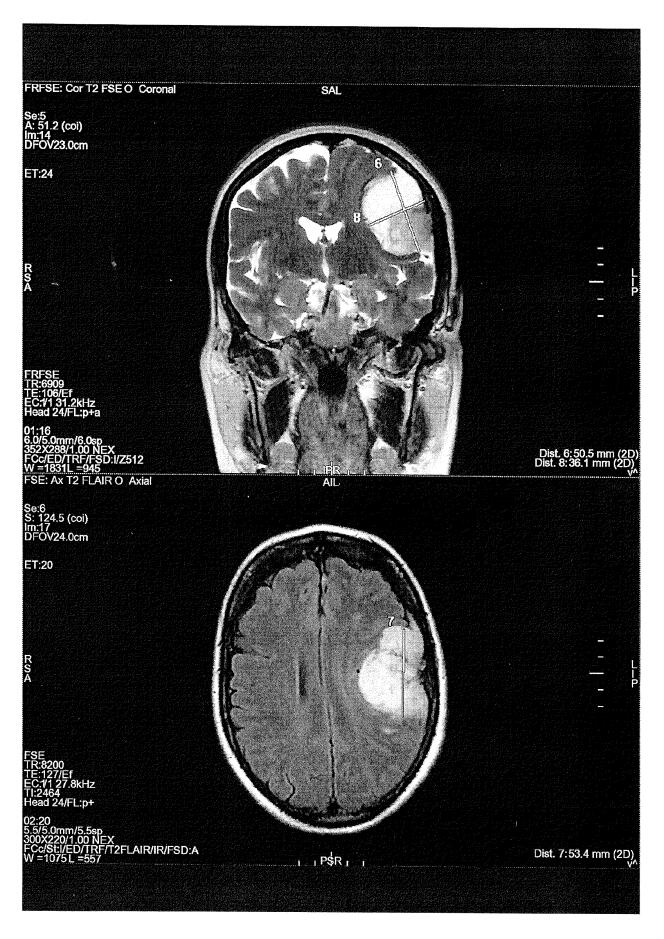
At the core of one of the two appeals, 50-year-old Céline A., who wishes to remain anonymous, took out an application between 2010 and 2016 on the advice of her gynecologist: “No one warned me that a simple pill could turn my life upside down. » On September 8, 2016 her life was “turned upside down”. Following a car accident and persistent neck pain and tremors in her left arm, she underwent magnetic resonance imaging (MRI) of the brain: the exam revealed three meningiomas. The largest, the size of a clementine, is removed immediately. Eight years later, she can no longer drive or work due to headaches, difficulty concentrating and balancing, and decreased visual acuity. and live together “The Sword of Damocles Over the Head” : Two meningiomas to monitor and “Fear” New hazardous operations.
Before being able to refer the matter to an administrative judge, Céline A. had to undergo a lengthy medical examination as part of the “summary evaluation” legal process. Final Report, October 2022, k the world were able to consult, leaving little room for doubt: “The causal link between pathology and presenting symptoms (…) And Androkar is known » And “Can’t be attributed to any other cause”. The document is equally clear on ANSM’s reaction: “It seems illogical that preventive measures were taken only from 2018” While the agency itself shows on its website that “The threat was established in 2009”. The first pharmacovigilance alert dates back to 2004, in which five cases of meningiomas were observed in patients treated with cyproterone acetate. And there was danger “Identified” According to experts in early 2007. That year, the first scientific publication, by neurosurgeon Sebastian Frölich, sounded the alarm and the Bayer laboratory itself reported. “High risk of meningiomas in users of cyproterone acetate”..
You have 57.64% of this article left to read. The rest is reserved for subscribers.




:quality(75)/cloudfront-us-east-1.images.arcpublishing.com/elcomercio/MCXAQ537IBEMJJUDIMCUCJT36E.jpg)