A new generation of CAR-T therapy drastically shrinks tumors within days
⇧ (video) You may also like this partner content
In a trial of a new version of CAR-T cell therapy, all patients experienced significant regression of their glioblastoma in just a few days. Designed to be more versatile than standard CAR-T, the treatment can target a broader population of tumor cells, significantly improving its effectiveness. Although the patients relapsed immediately after treatment, the researchers believe that the speed of early remission is already a great success.
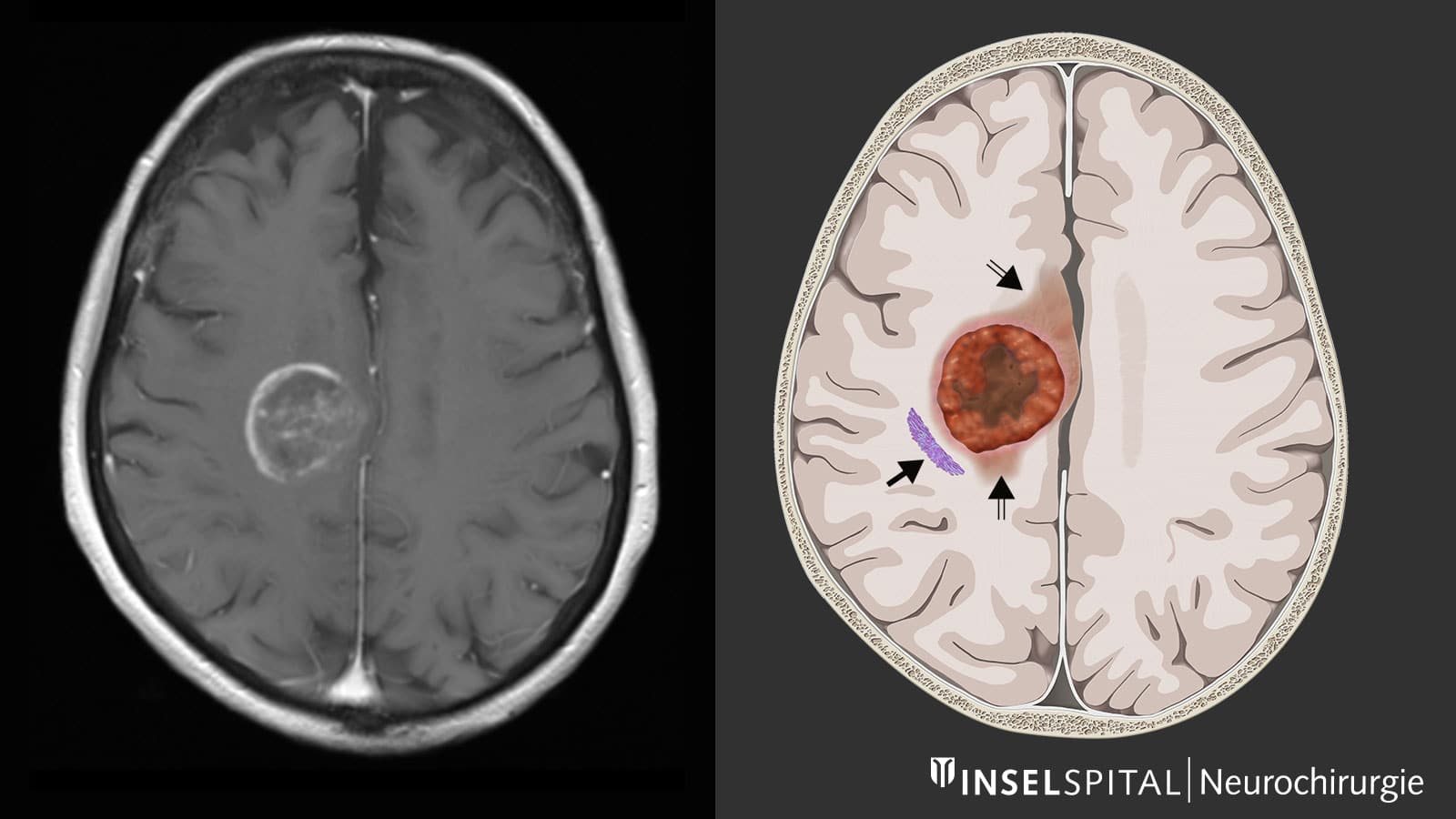
Glioblastoma (GBM) is the most invasive, aggressive and common tumor of the central nervous system. It arises from supporting brain cells and can develop in any part of the brain. It is estimated to affect about 3 in 100,000 people worldwide, usually between the ages of 50 and 70. In the absence of treatment, the 5-year survival rate usually does not exceed 7%.
Although the survival rate can be improved by resection of the tumor (by 80%), only its complete removal can best improve survival. Standard treatment consists of a combination of surgery, radiotherapy and chemotherapy (Stoop protocol), but the recurrence rate remains very high.
On the other hand, chimeric antigen receptor cell (CAR-T)-based therapy has shown promise for the treatment of malignancies such as refractory lymphoid tumors. Its effectiveness stems from its ability to be personalized for each patient. Indeed, this technique involves genetically modifying the patient’s T cells to develop antigenic recognition of the tumor cells and then re-introducing them into the patient.
It has been suggested that CAR-T therapy may also be used to treat GBM. However, its ability to target solid tumors is limited, mainly due to the heterogeneity of the cells that make them up. ” The CAR-T platform has revolutionized the way we think about treating cancer patients, but solid tumors like glioblastoma are difficult to treat because not all cancer cells are the same. », Brian D. Choi, a neurosurgeon at Massachusetts General Hospital, explained in a press release. In addition, solid tumors have a highly immunosuppressive tumor microenvironment.
To overcome these challenges, Choi and his team have proposed a new strategy combining both CAR-T therapy and bispecific antibodies. Also known as a T-cell engaging antibody molecule (TEAM), this is a type of antibody that binds to two targets instead of just one (like monoclonal antibodies). ” Our approach combines two forms of therapy, allowing us to treat glioblastoma more comprehensively and potentially more effectively. », the expert explains. The results of the study are available in the journal New England Journal of Medicine.
Up to 60% reduction in tumor volume
In an earlier study, Choi and colleagues developed CAR-T cells targeting a common cancer mutation called epidermal growth factor receptor variant 3 (EGFRv3). Although the strategy showed a positive response in GBM patients, no response was observed in recurrent tumor cells (responsible for recurrence). The latter express a wild-type EGFR mutation, which is not found in healthy tissue, but is expressed in more than 80% of GBM cases.
To overcome this obstacle, the researchers developed CARv3-TEAM-E cells, which both target EGFRv3 and induce the secretion of TEAM antibodies, which can recognize wild-type EGFR. The approach has shown promising results in preclinical models of GBM. TEAM antibodies produced by new versions of CAR-T cells induce responses at sites of high antigenic heterogeneity. Test in vitro also showed that these molecules can cause the activation of regulatory T cells, thereby improving the immune response against tumors.
See also

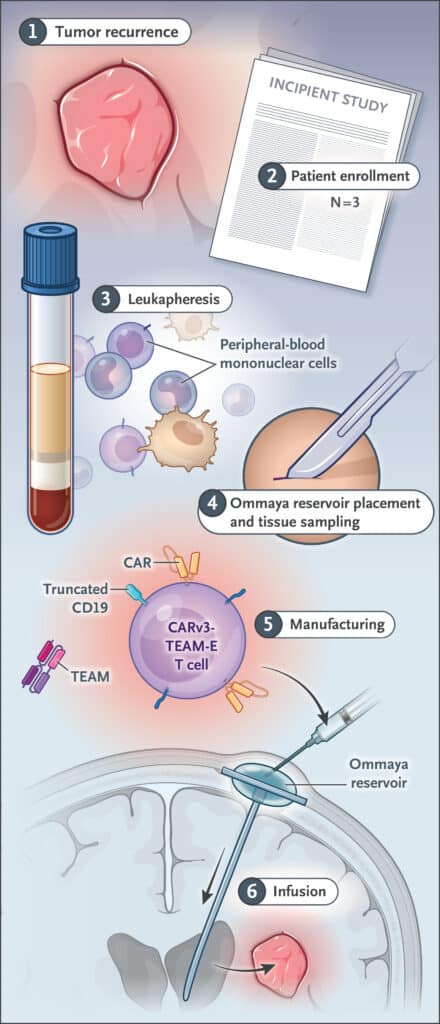
Diagram summarizing the treatment administration protocol. © Brian D. Choi et al.
The new study concerns a non-randomized phase 1 clinical trial including 3 patients aged 74, 72 and 57 years. All patients were initially treated with standard radiotherapy and temozolomide-based chemotherapy. They were included in the trial after recurrence. The new CARv3-TEAM-E treatment was delivered via an Ommaya reservoir, a type of intraventricular catheter that provides direct access to cerebrospinal fluid (CSF).
In a 74-year-old patient, after a single infusion of CARv3-TEAM-E, tumor volume shrank rapidly, but transiently. Their blood and CSF showed significant reductions in EGFRvIII and EGFR (wild-type) copy numbers. A 72-year-old patient showed an 18.5% reduction in tumor size, just 2 days after his infusion. at 69E At day, the tumor shrank by 60.7% and the response was maintained for more than 6 months. For the third patient (57 years old), the tumor almost completely disappeared after 5 days of treatment. Adverse effects reported are consistent with those commonly observed for CAR-T therapies (fever and altered mental status after infusion).
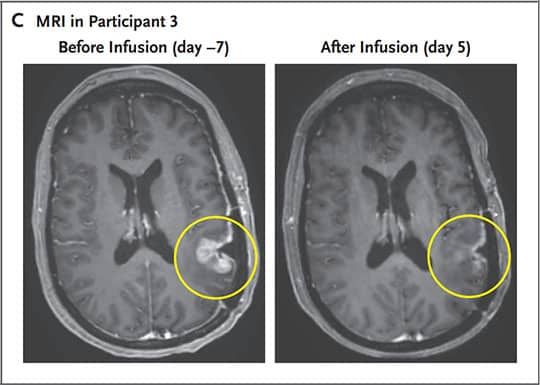
MRI of Participant 3 (age 57), showing a decrease in his tumor volume (circled in yellow). © Brian D. Choi et al.
However, although initial responses to treatment were remarkable, unfortunately all patients relapsed. According to experts, this is due to the limited persistence of CARv3-TEAM-E cells during the weeks following treatment — patients received only one infusion. ” We report a spectacular and rapid response in these three patients. Our work to date shows that we are making progress, but much remains to be done “, explains Elizabeth Gerstner, neuro-oncologist in the Department of Neurology at Massachusetts General Hospital and co-author of the study. As a next step, the team plans to combine serial infusions with chemotherapy in the hope of prolonging the treatment response.
Source: New England Journal of Medicine