Regenerative therapy for type 1 diabetes: an end to insulin dependence soon?
Despite advances in therapeutic strategies for the treatment of type 1 diabetes (T1D), exogenous insulin administration remains the routine for most patients. Indeed, if it is now possible to restore hormone production by transplantation, its widespread clinical application involves many challenges. Recent pharmacological approaches aimed at regenerating pancreatic β cells may be a game-changer. We spoke to an expert in hopes of finding out if this strategy could actually become available in the relatively near future.
Type 1 diabetes (T1D), or insulin-dependent diabetes, is characterized by autoimmune and irreversible destruction of the beta cells of the pancreas, which are responsible for the synthesis, release and storage of insulin. These cells represent approximately 50 to 70% of the total islet cell mass of the human pancreas. While symptoms usually do not appear until 80% of beta cells are destroyed, dysregulation of blood sugar leads to multiple long-term complications, such as diabetic nephropathy, neuropathy, retinopathy, cataracts, and cardiovascular disease.
An estimated 8.42 million people will suffer from T1D worldwide in 2021, with approximately 510,000 new cases per year. 60% of cases are concentrated in the 10 countries with the highest prevalence in the world, including the United States, India, Brazil, China, Germany, the United Kingdom, Russia, Canada, Saudi Arabia, and Spain. Recent modeling suggests that the annual prevalence of the disease could reach between 13.5 and 17.4 million by 2040, and that the greatest increase will be seen in low- and middle-income countries.
Excessive doses of insulin
The loss of beta cells means that patients with T1D must monitor their blood sugar levels daily and compensate for their insulin deficiency to maintain levels close to normal. To do this, first-line treatment usually involves daily insulin injections. Insulinotropic drugs (stimulating insulin production) may also be prescribed in certain cases.
” People with type 1 diabetes rely on injected or pumped insulin for the rest of their lives to control their blood sugar levels. », confirms in an e-mail
Trust my science Assam Al-Ousta, a distinguished epigeneticist at the Baker Heart and Diabetes Institute and Monash University (in Australia). However, “Although current therapeutic approaches for type 1 diabetes save lives, it is currently estimated that only one in five patients achieve meaningful blood sugar control with insulin injections,” he added in response to our questions.
Furthermore, insulin doses are not only expensive, but also unavailable in many parts of the world due to lack of supply. In 2020, the average cost per dose (all types of insulin combined) in the United States was $98.70, compared with $12 and $9.08 in Canada and France, respectively.
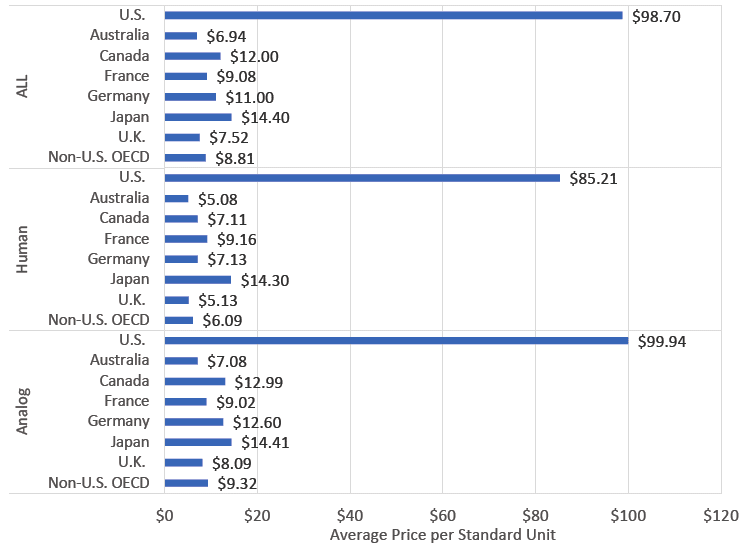
A chart comparing the average cost of insulin in 2018 in several countries around the world. © Mulcahy AW, et al. RAND Corporation, 2020
On the other hand, more metabolic control (for example through intensive insulin therapy) makes it possible to prevent or reduce the incidence of secondary complications associated with the disease. However, it still presents high risks of hypoglycemia and weight gain. Furthermore, although these techniques stabilize blood sugar levels, they cannot reverse or slow the destruction of beta cells.
Islet transplantation faces several limitations
The most advanced current treatments aimed at restoring beta cell function involve either whole pancreas transplantation or pancreatic islet (or islet of Langerhans) transplantation – a relatively less invasive procedure than the former. Unlike previous strategies, which only aimed to maintain blood sugar levels close to normal, transplantation aims to restore normoglycemia by restoring the endogenous and controlled secretion of insulin and other hormones produced by the islets of Langerhans.
A 90% success rate of one-year viability of islet allografts has been reported by various health centers over the past two decades. However, this option faces significant challenges for its widespread use. Indeed, pancreatic islet transplantation involves isolating grafts from the pancreas of a deceased donor, then injecting them under immunosuppression through the portal vein. Although in some cases islets have been collected from living donors (via partial pancreatectomy), the postoperative complication rate is so high that this method is rarely chosen.
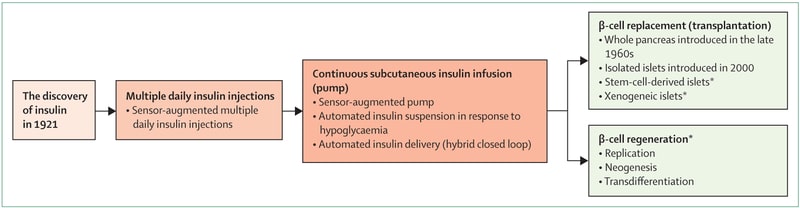
Major advances in the treatment of T1D, from the discovery of insulin to the latest regenerative therapies. © Marie-Christine Vantigame et al.
Translation of the main box:
- The discovery of insulin
- Multi-day insulin injection
- Continuous subcutaneous insulin infusion (pump)
- (Top) Beta cell replacement by transplantation
- (Bottom) Beta cell regeneration
On the other hand, “despite their proven clinical utility, these therapies face the harsh reality of donor shortages as well as the associated side effects of immunosuppressive drugs,” Al-Aosta explains. Trust my science. Indeed, insulin independence requires 2 to 3 infusions with a target of 9000 islets per kilogram of recipient body weight. Approximately 3 pancreases are needed to produce enough islets for transplantation into a recipient. On the other hand, the cost of the intervention (which is carried out by only a few specialized institutions in the world), from islet isolation to transplantation and postoperative follow-ups, is considerable.
To overcome these challenges, various potential sources are being investigated for the purpose of producing large quantities of beta cells. The cells, for example, arise from the differentiation of human or embryonic pluripotent stem cells. A variety of molecules including soluble growth and transcription factors have been used for this purpose. However, the poor reproducibility of differentiation protocols remains a major challenge. Indeed, resuscitation efforts have so far been relatively risky.
Regenerative drugs have already been approved by the FDA
Given the difficulties associated with previous strategies, there is an urgent need to identify new treatments that stimulate growth and restore beta cell function. In this vision, pharmacological approaches have recently been explored for reactivation or regeneration at the original location From these cells.



:focal(1979x1260:1989x1250):watermark(cloudfront-eu-central-1.images.arcpublishing.com/ipmgroup/OIO44DUUUNC2DB5T3FIJECBT5U.png,0,-0,0,100)/cloudfront-eu-central-1.images.arcpublishing.com/ipmgroup/L3VX5LQTGNEDTIJN6PHR4RYC3A.jpg)

