Short toxic RNA strands may be involved in neuron loss
⇧ (video) You may also like this partner content
A recent study suggests that short strands of toxic RNA are involved in the self-destruction of neurons in Alzheimer’s disease through a process called “RNA interference.” These strands are found in abnormally high amounts in the brains of Alzheimer’s patients as well as in the brains of elderly individuals. The discovery may pave the way for new therapeutic approaches, which may extend to other neurodegenerative diseases as well.
According to the conventional hypothesis, Alzheimer’s is a neurodegenerative disease caused by a sequence of events known as the amyloid cascade. It is particularly characterized by the accumulation of beta-amyloid plaques and tangles of tau protein, which lead to progressive loss of neurons. However, this theory has recently been called into question and it is still difficult to identify the exact chain of events leading to the death of neurons.
Although the pathophysiology of the disease lacks precision, 70-80% of its treatment efforts focus on reducing amyloid plaques. However, treatment based on this strategy lacks efficacy and only slows the progression of the disease. This has led to the discovery of new approaches and potential therapeutic targets.
It has recently been suggested that DNA damage associated with aging causes the accumulation of somatic changes in people suffering from Alzheimer’s disease. With all the genetic information necessary for our biological functions stored in DNA… To convert this genetic information into the building blocks of life, DNA must be converted into RNA, which is used by the cellular machinery to produce proteins.
Apart from protein-coding RNAs, there are short versions (RNAs) that are non-coding and provide other essential functions. Among these functions is RNA interference, which leads to the inactivation of proteins that code for “long” RNA. It is, among other things, a form of post-transcriptional regulation that negatively regulates gene expression at the coding RNA level.
Researchers at Northwestern University in the United States have suggested that RNA interference may be involved in the etiology of Alzheimer’s. As part of their new study published in the journal Nature Communications, they specifically identified toxic RNAs that damage DNA, leading to neuronal loss in Alzheimer’s patients and the elderly. Normally, these strands are regulated by so-called “protective” RNAs.
” No one has ever linked RNA activities to Alzheimer’s disease ” Corresponding author of the study, Marcus Peter of Northwestern University, said in a statement. We found that in aged brain cells, the balance between toxic and protective RNAs is tipped toward toxic RNAs. “, he explained.
Imbalance between protective and toxic RNA
Micro-RNA (a type of RNA) provides several regulatory functions at the cell level and ensures their survival by playing a protective role. They are actually a type of protector that prevents toxic RNA from inducing RNA interference that can damage the cell.
During their studies, Peter and his colleagues identified RNA sequences that can destroy cells by blocking the production of proteins necessary for their survival. This is a process called death-induced by survival gene elimination (DISE), which is involved in the elimination of tumor cells. Researchers have suggested that it may also induce neuron loss in Alzheimer’s disease.
To test their hypothesis, the experts analyzed the behavior of RNA in the brains of mouse models of Alzheimer’s, in the brains of young and old mice, in neurons derived from induced pluripotent stem cells of healthy individuals. Health (young and old) and patients. Suffering from Alzheimer’s. Multiple neuron-like cell lines derived from human brain and treated with beta-amyloid fragments were also analyzed. The brains of a group of “superelders” aged at least 80 were also examined. These are old people whose memory is equivalent to a person 20 to 30 years younger.
See also

Researchers have discovered a strong link between DISE, DNA damage and neuronal loss in Alzheimer’s disease and aging. Levels of protective RNA were significantly decreased in disease and aging models. In contrast, the data showed that levels of protective microRNAs were significantly higher in the brains of super-elders. These results suggest that lower levels of these RNAs in the aged brain allow toxic RNAs to invade neurons and induce interference.
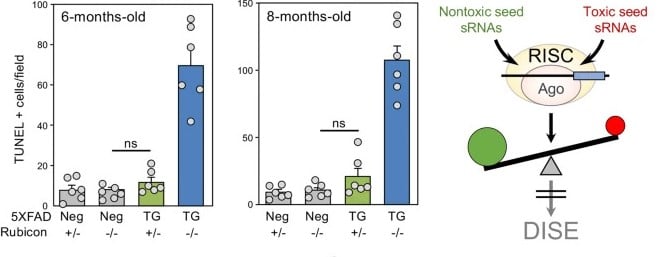
Left, Quantification of TUNEL (DNA fragmentation) positivity in mouse brains of four different genotypes. A 6-month-old mouse on the left and an 8-month-old mouse on the right. Right, diagram illustrating how the ratio between non-toxic (green) and toxic (red) RNA can protect cells from DISE. © Bidur Paudel et al.
Additionally, “Our data provide a new explanation for why, in almost all neurodegenerative diseases, affected individuals live for decades with no (or few) symptoms, and then the disease gradually takes hold as cells lose their protection as they age. ,” suggests Peter. . It is also interesting to note that Alzheimer’s patients have a surprisingly low rate of cancer compared to the average – consistent with the DISE overactivation hypothesis.
On the other hand, experience in vitro showed that cells exposed to amyloid protein showed more DNA damage, which is associated with toxic RNA-induced interference. By increasing the amount of protective sRNA, these neurons appear to be less susceptible to DNA damage. In addition, enhancing protein activity to increase levels of protective microRNAs completely abolished the DNA damage mechanism and partially inhibited DISE.
These results suggest that increasing levels of protective microRNAs may be a new therapeutic approach for both Alzheimer’s and other neurodegenerative diseases. The next step in research is to explore this pathway and identify the best compounds to selectively increase the levels of protective sRNAs or block their sRNA counterparts.
Source: Nature Communications





